Dosage and
Administration for
ProQuad® (Measles,
Mumps, Rubella and
Varicella Virus Vaccine
Live)

Actor Portrayal
Dosage
A single dose of ProQuad is approximately 0.5 mL and is administered intramuscularly or subcutaneously. The first dose is usually administered at 12 to 15 months of age but may be given anytime through 12 years of age.
The second dose is usually administered at 4 to 6 years of age. At least 1 month should elapse between a dose of a measles-containing vaccine, such as M-M-R®II (Measles, Mumps, and Rubella Virus Vaccine Live), and a dose of ProQuad. At least 3 months should elapse between a dose of varicella-containing vaccine and ProQuad.


Actor Portrayal
Flexible Route of Administration
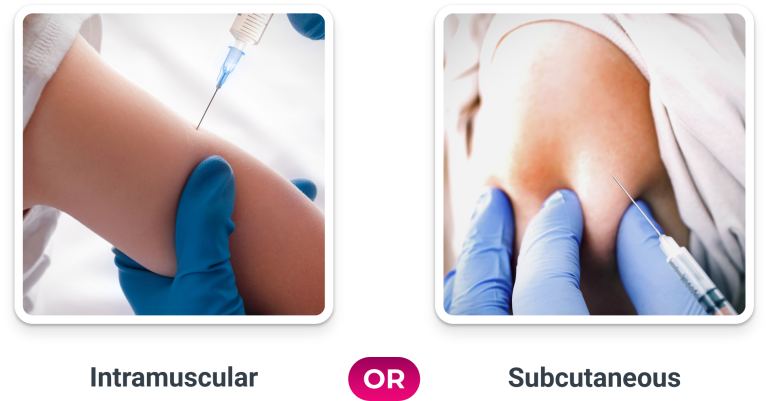
Not actual administration of ProQuad. For illustration purposes only.
You have the option to choose to administer all routinely recommended injectable pediatric vaccinations included in the CDC immunization schedule via the same IM route when you choose ProQuad as your measles, mumps, rubella, and varicella vaccine.
Method of Administration
Inject the vaccine intramuscularly or subcutaneously into the outer aspect of the deltoid region of the upper arm or into the higher anterolateral area of the thigh.
Indications and Usage for ProQuad
ProQuad is a vaccine indicated for active immunization for the prevention of measles, mumps, rubella, and varicella in children 12 months through 12 years of age.
VARIVAX is a vaccine indicated for active immunization for the prevention of varicella in individuals 12 months of age or older.
M-M-R®II is indicated for active immunization for the prevention of measles, mumps, and rubella in individuals 12 months of age or older.
Selected Safety Information for ProQuad
- Hypersensitivity: ProQuad, M-M-R®II, and VARIVAX are contraindicated in patients with a history of anaphylactic reaction or hypersensitivity to any component of the vaccine (including gelatin or neomycin) or to a prior dose of measles, mumps, rubella, or varicella-containing vaccine. Use caution when administering ProQuad and M-M-R®II to individuals with anaphylaxis or immediate hypersensitivity to eggs.
- ProQuad, M-M-R®II, and VARIVAX are contraindicated in certain individuals, including those with: immunodeficiency or who are immunosuppressed; an active febrile illness; untreated tuberculosis.
- Pregnancy: ProQuad, M-M-R®II, and VARIVAX are contraindicated for use in pregnant women. Do not administer ProQuad or VARIVAX to individuals who are planning to become pregnant in the next 3 months. Do not administer M-M-R®II to individuals who are planning to become pregnant in the next month.
- Febrile Seizures: Administration of ProQuad (dose 1) to children 12 to 23 months old who have not been previously vaccinated against measles, mumps, rubella, or varicella, nor had a history of the wild-type infections, is associated with higher rates of fever and febrile seizures at 5 to 12 days after vaccination when compared to children vaccinated with a first dose of both M-M-R®II and VARIVAX administered concomitantly.
- Febrile Seizures: Use caution when administering ProQuad and M-M-R®II to individuals with a history of febrile seizures.
- Family History of Immunodeficiency: Vaccination with ProQuad, M-M-R®II, and VARIVAX should be deferred in individuals with a family history of congenital or hereditary immunodeficiency until the individual’s immune status has been evaluated and the individual has been found to be immunocompetent.
- Thrombocytopenia: Transient thrombocytopenia has been reported within 4-6 weeks following vaccination with measles, mumps, and rubella vaccine. Carefully evaluate the potential risk and benefit of vaccination in children with thrombocytopenia or in those who experienced thrombocytopenia after vaccination with a previous dose of a measles, mumps, and rubella-containing vaccine.
- Varicella Transmission and Precautions: Advise vaccinees administered ProQuad or VARIVAX to avoid: close contact with high-risk individuals susceptible to varicella for up to 6 weeks following vaccination since transmission of varicella vaccine virus to susceptible contacts has been reported. Varicella vaccine virus transmission may occur between vaccine recipients and contacts susceptible to varicella including healthy individuals.
- Immune Globulins and Transfusions: Immune Globulins and other blood products should not be given concomitantly with ProQuad, M-M-R®II, or VARIVAX.
- Use of Salicylates: Avoid use of salicylates in children and adolescents administered ProQuad or VARIVAX for 6 weeks following vaccination due to the association of Reye Syndrome with salicylate therapy and wild-type varicella infection.
- Adverse Events: The following adverse events have been reported for both subcutaneous and intramuscular injections of ProQuad, M-M-R®II, and VARIVAX: fever, injection-site reactions (pain/tenderness/soreness, erythema, and swelling); and rash on the body or at the injection site. Additionally, irritability has been reported for the subcutaneous injections of ProQuad, M-M-R®II, and VARIVAX.
- ProQuad Systemic Vaccine-Related Adverse Events: Systemic vaccine-related adverse events that were reported at a significantly greater rate in recipients of subcutaneous ProQuad than in recipients of the component vaccines administered concomitantly were fever and measles-like rash.
- VARIVAX Dose-related Adverse Events: In a clinical trial involving children who received 2 doses of VARIVAX 3 months apart, the incidence of injection-site clinical complaints observed in the first 4 days following vaccination was slightly higher post-dose 2 (overall incidence 25.4%) than post-dose 1 (overall incidence 21.7%), whereas the incidence of systemic clinical complaints in the 42-day follow-up period was lower post-dose 2 (66.3%) than post-dose 1 (85.8%).
- Concomitant Vaccines With ProQuad: ProQuad may be administered concomitantly with diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed (DTaP), Haemophilus influenzae type b conjugate (meningococcal protein conjugate) and hepatitis B (recombinant) vaccine. It may also be administered concomitantly with pneumococcal 7-valent conjugate vaccine and/or hepatitis A vaccine (inactivated) at separate injection sites.
- Concomitant Vaccines With VARIVAX: VARIVAX can be administered with other live viral vaccines. If not given concurrently, at least 1 month should elapse between a dose of a live attenuated measles virus-containing vaccine and a dose of VARIVAX. In children, at least 3 months should elapse between administration of 2 doses of a live attenuated varicella virus-containing vaccine.
- Tuberculin Testing: If a tuberculin test is to be done with M-M-R®II and ProQuad, it should be administered either any time before, simultaneously with, or at least 4 to 6 weeks after vaccination. With VARIVAX, tuberculin testing may be performed before the vaccine is administered or at least 4 weeks following vaccination.
- Additional M-M-R®II Precautions: Additional adverse reactions, which have been reported without regard to causality, include febrile convulsions, arthritis, thrombocytopenia, anaphylaxis, anaphylactoid reactions, arthritis, encephalitis and encephalopathy in their diverse clinical presentations.
- Additional VARIVAX Precautions: It is not known if varicella vaccine virus is excreted in human milk. A boost in antibody levels has been observed in vaccinees following exposure to wild-type varicella, which could account for the apparent long-term persistence of antibody levels in studies. The duration of protection from varicella infection after vaccination is unknown.
- ProQuad/VARIVAX and Herpes Zoster: The long-term effect of VARIVAX on the incidence of herpes zoster, particularly in those vaccinees exposed to wild-type varicella, is unknown at present.
- Efficacy: Vaccination with ProQuad, VARIVAX, or M-M-R®II may not result in protection in 100% of vaccinees.
Dosage and Administration
ProQuad:
- Each dose of ProQuad is approximately 0.5 mL and is administered intramuscularly or subcutaneously.
- At least 1 month should elapse between a dose of a measles-containing vaccine such as M-M-R®II and a dose of ProQuad. At least 3 months should elapse between a dose of varicella-containing vaccine and ProQuad.
VARIVAX:
- Each dose is approximately 0.5 mL and is administered intramuscularly or subcutaneously.
- The first dose is administered at 12 to 15 months of age.
- The second dose is administered at 4 to 6 years of age.
- There should be a minimum interval of 3 months between doses.
- 12 months to 12 years of age: If a second dose is administered, there should be a minimum interval of 3 months between doses.
- Adolescents (≥13 years of age) and Adults: 2 doses, to be administered with a minimum interval of 4 weeks between doses.
M-M-R®II:
- The dose for any age is approximately 0.5 mL administered intramuscularly or subcutaneously.
- The recommended age for primary vaccination is 12 to 15 months and the second dose should be given at 4 to 6 years of age.
Before administering VARIVAX® (Varicella Virus Vaccine Live), M-M-R®II (Measles, Mumps, and Rubella Virus Vaccine Live), or ProQuad® (Measles, Mumps, Rubella and Varicella Virus Vaccine Live), please read the accompanying Prescribing Information. The Patient Information also is available for VARIVAX, M-M-R®II, and ProQuad.
Indications and Usage for ProQuad
ProQuad is a vaccine indicated for active immunization for the prevention of measles, mumps, rubella, and varicella in children 12 months through 12 years of age.
VARIVAX is a vaccine indicated for active immunization for the prevention of varicella in individuals 12 months of age or older.
M-M-R®II is indicated for active immunization for the prevention of measles, mumps, and rubella in individuals 12 months of age or older.
ProQuad is a vaccine indicated for active immunization for the prevention of measles, mumps,
ProQuad is a vaccine indicated for active immunization for the prevention of measles, mumps, rubella, and varicella in children 12 months through 12 years of age.
VARIVAX is a vaccine indicated for active immunization for the prevention of varicella in individuals 12 months of age or older.
Selected Safety Information for ProQuad
- Hypersensitivity: ProQuad, M-M-R®II, and VARIVAX are contraindicated in patients with a history of anaphylactic reaction or hypersensitivity to any component of the vaccine (including gelatin or neomycin) or to a prior dose of measles, mumps, rubella, or varicella-containing vaccine. Use caution when administering ProQuad and M-M-R®II to individuals with anaphylaxis or immediate hypersensitivity to eggs.
- ProQuad, M-M-R®II, and VARIVAX are contraindicated in certain individuals, including those with: immunodeficiency or who are immunosuppressed; an active febrile illness; untreated tuberculosis.
- Pregnancy: ProQuad, M-M-R®II, and VARIVAX are contraindicated for use in pregnant women. Do not administer ProQuad or VARIVAX to individuals who are planning to become pregnant in the next 3 months. Do not administer M-M-R®II to individuals who are planning to become pregnant in the next month.
- Febrile Seizures: Administration of ProQuad (dose 1) to children 12 to 23 months old who have not been previously vaccinated against measles, mumps, rubella, or varicella, nor had a history of the wild-type infections, is associated with higher rates of fever and febrile seizures at 5 to 12 days after vaccination when compared to children vaccinated with a first dose of both M-M-R®II and VARIVAX administered concomitantly.
- Febrile Seizures: Use caution when administering ProQuad and M-M-R®II to individuals with a history of febrile seizures.
- Family History of Immunodeficiency: Vaccination with ProQuad, M-M-R®II, and VARIVAX should be deferred in individuals with a family history of congenital or hereditary immunodeficiency until the individual’s immune status has been evaluated and the individual has been found to be immunocompetent.
- Thrombocytopenia: Transient thrombocytopenia has been reported within 4-6 weeks following vaccination with measles, mumps, and rubella vaccine. Carefully evaluate the potential risk and benefit of vaccination in children with thrombocytopenia or in those who experienced thrombocytopenia after vaccination with a previous dose of a measles, mumps, and rubella-containing vaccine.
- Varicella Transmission and Precautions: Advise vaccinees administered ProQuad or VARIVAX to avoid: close contact with high-risk individuals susceptible to varicella for up to 6 weeks following vaccination since transmission of varicella vaccine virus to susceptible contacts has been reported. Varicella vaccine virus transmission may occur between vaccine recipients and contacts susceptible to varicella including healthy individuals.
- Immune Globulins and Transfusions: Immune Globulins and other blood products should not be given concomitantly with ProQuad, M-M-R®II, or VARIVAX.
- Use of Salicylates: Avoid use of salicylates in children and adolescents administered ProQuad or VARIVAX for 6 weeks following vaccination due to the association of Reye Syndrome with salicylate therapy and wild-type varicella infection.
- Adverse Events: The following adverse events have been reported for both subcutaneous and intramuscular injections of ProQuad, M-M-R®II, and VARIVAX: fever, injection-site reactions (pain/tenderness/soreness, erythema, and swelling); and rash on the body or at the injection site. Additionally, irritability has been reported for the subcutaneous injections of ProQuad, M-M-R®II, and VARIVAX.
- ProQuad Systemic Vaccine-Related Adverse Events: Systemic vaccine-related adverse events that were reported at a significantly greater rate in recipients of subcutaneous ProQuad than in recipients of the component vaccines administered concomitantly were fever and measles-like rash.
- VARIVAX Dose-related Adverse Events: In a clinical trial involving children who received 2 doses of VARIVAX 3 months apart, the incidence of injection-site clinical complaints observed in the first 4 days following vaccination was slightly higher post-dose 2 (overall incidence 25.4%) than post-dose 1 (overall incidence 21.7%), whereas the incidence of systemic clinical complaints in the 42-day follow-up period was lower post-dose 2 (66.3%) than post-dose 1 (85.8%).
- Concomitant Vaccines With ProQuad: ProQuad may be administered concomitantly with diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed (DTaP), Haemophilus influenzae type b conjugate (meningococcal protein conjugate) and hepatitis B (recombinant) vaccine. It may also be administered concomitantly with pneumococcal 7-valent conjugate vaccine and/or hepatitis A vaccine (inactivated) at separate injection sites.
- Concomitant Vaccines With VARIVAX: VARIVAX can be administered with other live viral vaccines. If not given concurrently, at least 1 month should elapse between a dose of a live attenuated measles virus-containing vaccine and a dose of VARIVAX. In children, at least 3 months should elapse between administration of 2 doses of a live attenuated varicella virus-containing vaccine.
- Tuberculin Testing: If a tuberculin test is to be done with M-M-R®II and ProQuad, it should be administered either any time before, simultaneously with, or at least 4 to 6 weeks after vaccination. With VARIVAX, tuberculin testing may be performed before the vaccine is administered or at least 4 weeks following vaccination.
- Additional M-M-R®II Precautions: Additional adverse reactions, which have been reported without regard to causality, include febrile convulsions, arthritis, thrombocytopenia, anaphylaxis, anaphylactoid reactions, arthritis, encephalitis and encephalopathy in their diverse clinical presentations.
- Additional VARIVAX Precautions: It is not known if varicella vaccine virus is excreted in human milk. A boost in antibody levels has been observed in vaccinees following exposure to wild-type varicella, which could account for the apparent long-term persistence of antibody levels in studies. The duration of protection from varicella infection after vaccination is unknown.
- ProQuad/VARIVAX and Herpes Zoster: The long-term effect of VARIVAX on the incidence of herpes zoster, particularly in those vaccinees exposed to wild-type varicella, is unknown at present.
- Efficacy: Vaccination with ProQuad, VARIVAX, or M-M-R®II may not result in protection in 100% of vaccinees.
Dosage and Administration
ProQuad:
- Each dose of ProQuad is approximately 0.5 mL and is administered intramuscularly or subcutaneously.
- At least 1 month should elapse between a dose of a measles-containing vaccine such as M-M-R®II and a dose of ProQuad. At least 3 months should elapse between a dose of varicella-containing vaccine and ProQuad.
VARIVAX:
- Each dose is approximately 0.5 mL and is administered intramuscularly or subcutaneously.
- The first dose is administered at 12 to 15 months of age.
- The second dose is administered at 4 to 6 years of age.
- There should be a minimum interval of 3 months between doses.
- 12 months to 12 years of age: If a second dose is administered, there should be a minimum interval of 3 months between doses.
- Adolescents (≥13 years of age) and Adults: 2 doses, to be administered with a minimum interval of 4 weeks between doses.
M-M-R®II:
- The dose for any age is approximately 0.5 mL administered intramuscularly or subcutaneously.
- The recommended age for primary vaccination is 12 to 15 months and the second dose should be given at 4 to 6 years of age.
Before administering VARIVAX® (Varicella Virus Vaccine Live), M-M-R®II (Measles, Mumps, and Rubella Virus Vaccine Live), or ProQuad® (Measles, Mumps, Rubella and Varicella Virus Vaccine Live), please read the accompanying Prescribing Information. The Patient Information also is available for VARIVAX, M-M-R®II, and ProQuad.
Hypersensitivity: ProQuad, M-M-R®II, and VARIVAX are contraindicated in patients with a
Hypersensitivity: ProQuad, M-M-R®II, and VARIVAX are contraindicated in patients with a history of anaphylactic reaction or hypersensitivity to any component of the vaccine (including gelatin or neomycin) or to a prior dose of measles, mumps, rubella, or varicella-containing vaccine. Use caution when administering ProQuad and M-M-R®II to individuals
