Pediatric Flexible Dosing Schedule
Available as a prefilled syringe
Not shown actual size
NDC 0006-4095-02
Flexible dosing window: VAQTA offers 6 more months compared to Havrix*,1
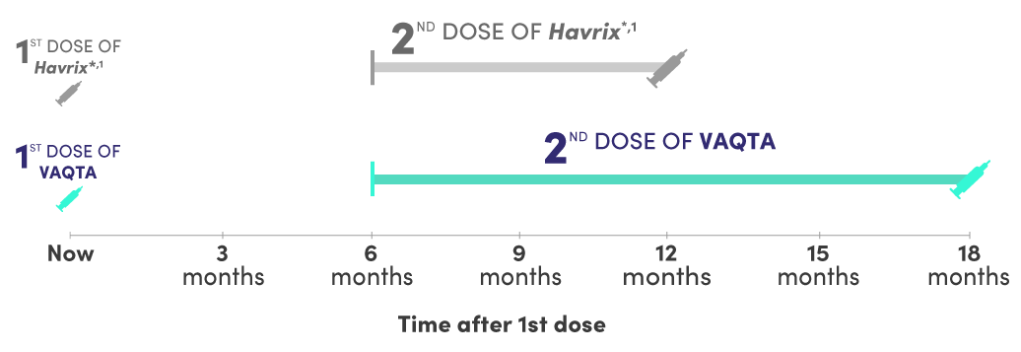
Interchangeability
VAQTA can be administered 6 to 12 months following a first dose of Havrix*
*Havrix is a registered trademark of GlaxoSmithKline.
ACIP catch-up vaccination recommendation2
Two-dose series for all children and adolescents aged 2–18 years not previously vaccinated (minimum interval: 6 months).
Patients who previously received 1 dose at age 12 months or older should receive dose 2 at least 6 months after dose 1.2
Catch-up Vaccination Opportunities
bMost vaccines can be administered in cases of mild illness, after screening for contraindications and weighing the risks and benefits.6
Reference
Reference
Reference
Reference
Reference
Concomitant Dosing
Reference
Reference
Reference
Reference
Reference
Reference
Indication for VAQTA
VAQTA is indicated for the prevention of disease caused by hepatitis A virus (HAV) in persons 12 months of age and older. The primary dose should be given at least 2 weeks prior to expected exposure to HAV.
Dosage and Administration for VAQTA
Children/Adolescents (12 months through 18 years of age): The vaccination schedule consists of a primary 0.5 mL dose administered intramuscularly and a 0.5 mL booster dose administered intramuscularly 6 to 18 months later.
Booster Immunization Following Another Manufacturer’s Hepatitis A Vaccine: A booster dose of VAQTA may be given at 6 to 12 months following a primary dose of Havrix*.
*Havrix is a registered trademark of GlaxoSmithKline.
Select Safety Information for VAQTA
Do not administer VAQTA to individuals with a history of immediate and/or severe allergic or hypersensitivity reactions (eg, anaphylaxis) after a previous dose of any hepatitis A vaccine, or to individuals who have had an anaphylactic reaction to any component of VAQTA, including neomycin.
The vial stopper and the syringe plunger stopper and tip cap contain dry natural latex rubber that may cause allergic reactions in latex-sensitive individuals.
The most common local adverse reactions and systemic adverse events (≥15%) reported in different clinical trials across different age groups when VAQTA was administered alone or concomitantly were:
- Children 12 through 23 months of age: injection-site pain/tenderness (37.0%), injection-site erythema (21.2%), and fever (16.4% when administered alone, and 27.0% when administered concomitantly).
- Children/Adolescents 2 through 18 years of age: injection-site pain (18.7%).
Safety and effectiveness in infants below 12 months of age have not been established.
Immunocompromised persons, including individuals receiving immunosuppressive therapy, may have a diminished immune response to VAQTA and may not be protected against HAV infection after vaccination.
Hepatitis A virus has a relatively long incubation period (approximately 20 to 50 days). VAQTA may not prevent hepatitis A infection in individuals who have an unrecognized hepatitis A infection at the time of vaccination.
In clinical trials in children, VAQTA was concomitantly administered with one or more of the following US-licensed vaccines: Measles, Mumps, and Rubella Virus Vaccine, Live; Varicella Vaccine, Live; Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine, Adsorbed; Measles, Mumps, Rubella, and Varicella Vaccine, Live; Pneumococcal 7-valent Conjugate Vaccine; and Haemophilus b Conjugate Vaccine (Meningococcal Protein Conjugate). Safety and immunogenicity were similar for concomitantly administered vaccines compared to separately administered vaccines.
The total duration of the protective effect of VAQTA in healthy vaccinees is unknown at present.
Vaccination with VAQTA may not result in a protective response in all susceptible vaccinees.
Before administering VAQTA, please read the accompanying Prescribing Information. The Patient Information also is available.
Indication for VAQTA
VAQTA is indicated for the prevention of disease caused by hepatitis A virus (HAV) in persons 12 months of age and older. The primary dose should be given at least 2 weeks prior to expected exposure to HAV.
Dosage and Administration for VAQTA
Children/Adolescents (12 months through 18 years of age): The vaccination schedule consists of a primary 0.5 mL dose administered intramuscularly and a 0.5 mL booster dose administered intramuscularly 6 to 18 months later.
Booster Immunization Following Another Manufacturer’s Hepatitis A Vaccine: A booster dose of VAQTA may be given at 6 to 12 months following a primary dose of Havrix*.
*Havrix is a registered trademark of GlaxoSmithKline.
VAQTA is indicated for the prevention of disease caused by hepatitis A
VAQTA is indicated for the prevention of disease caused by hepatitis A virus (HAV) in persons 12 months of age and older. The primary dose should be given at least 2 weeks prior to expected exposure to HAV.
Select Safety Information for VAQTA
Do not administer VAQTA to individuals with a history of immediate and/or severe allergic or hypersensitivity reactions (eg, anaphylaxis) after a previous dose of any hepatitis A vaccine, or to individuals who have had an anaphylactic reaction to any component of VAQTA, including neomycin.
The vial stopper and the syringe plunger stopper and tip cap contain dry natural latex rubber that may cause allergic reactions in latex-sensitive individuals.
The most common local adverse reactions and systemic adverse events (≥15%) reported in different clinical trials across different age groups when VAQTA was administered alone or concomitantly were:
- Children 12 through 23 months of age: injection-site pain/tenderness (37.0%), injection-site erythema (21.2%), and fever (16.4% when administered alone, and 27.0% when administered concomitantly).
- Children/Adolescents 2 through 18 years of age: injection-site pain (18.7%).
Safety and effectiveness in infants below 12 months of age have not been established.
Immunocompromised persons, including individuals receiving immunosuppressive therapy, may have a diminished immune response to VAQTA and may not be protected against HAV infection after vaccination.
Hepatitis A virus has a relatively long incubation period (approximately 20 to 50 days). VAQTA may not prevent hepatitis A infection in individuals who have an unrecognized hepatitis A infection at the time of vaccination.
In clinical trials in children, VAQTA was concomitantly administered with one or more of the following US-licensed vaccines: Measles, Mumps, and Rubella Virus Vaccine, Live; Varicella Vaccine, Live; Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine, Adsorbed; Measles, Mumps, Rubella, and Varicella Vaccine, Live; Pneumococcal 7-valent Conjugate Vaccine; and Haemophilus b Conjugate Vaccine (Meningococcal Protein Conjugate). Safety and immunogenicity were similar for concomitantly administered vaccines compared to separately administered vaccines.
The total duration of the protective effect of VAQTA in healthy vaccinees is unknown at present.
Vaccination with VAQTA may not result in a protective response in all susceptible vaccinees.
Before administering VAQTA, please read the accompanying Prescribing Information. The Patient Information also is available.
Do not administer VAQTA to individuals with a history of immediate and/or
Do not administer VAQTA to individuals with a history of immediate and/or severe allergic or hypersensitivity reactions (eg, anaphylaxis) after a previous dose of any hepatitis A vaccine, or to individuals who have had an anaphylactic reaction to any component of VAQTA, including neomycin.