aBefore 1989, people usually only received one dose of the measles vaccine or relied on natural immunity. Due to outbreaks, the CDC revised its recommendation for routine measles vaccination to a 2-dose program.2,3
Which adult populations are recommended by the CDC to get the MMR vaccine, and how many doses do they need?1
- Adults who do not have presumptive evidence of immunityb should get at least one dose of MMR vaccine.1
- Certain adults may need 2 doses1:
- Adults who are going to be in a setting that poses a high risk for measles transmission should make sure they have had two total doses separated by at least 28 days. These adults include1,4:


bPresumptive evidence of immunity can be established by written documentation of one or more doses of a measles-containing vaccine (two doses of measles-containing vaccine for school-aged children and adults at high risk), laboratory evidence of immunity, or birth before 1957.1
cHealth care personnel born before 1957 without laboratory evidence of immunity or disease should consider getting two doses of MMR vaccine.1
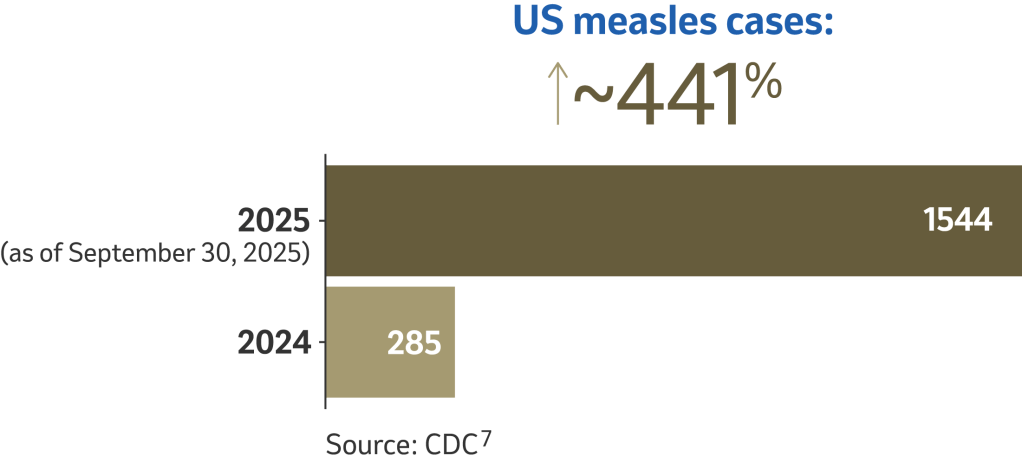
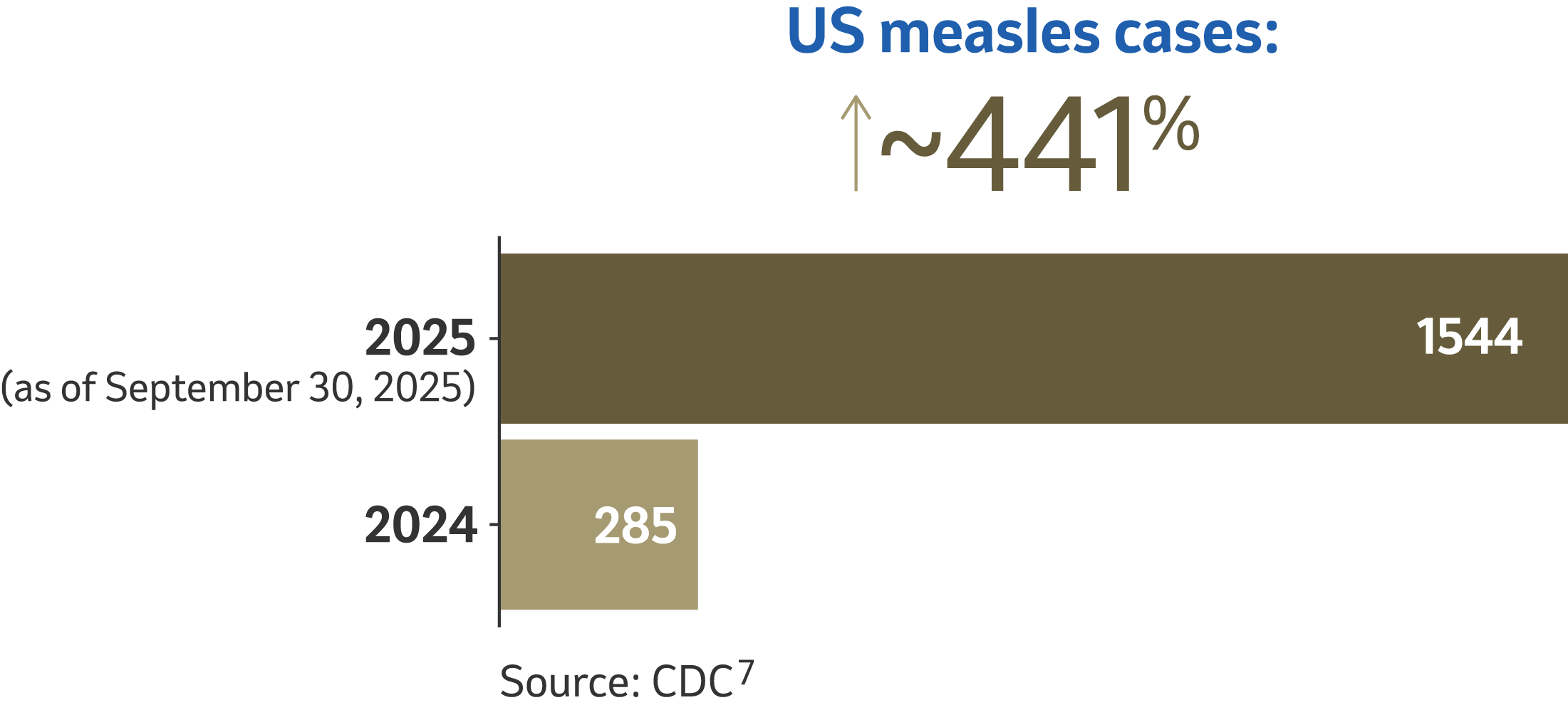
Measles cases and outbreaks still occur4
In the United States, where endemic measles has been declared eliminated, cases and outbreaks can still occur.5
The disease is brought into the United States by unvaccinated people who get infected in other countries. Typically, 2 out of 3 of these unvaccinated travelers are Americans. They can spread measles to other people who are not protected against measles, which can sometimes lead to outbreaks.4 Prolonged outbreaks can threaten the US measles elimination status.6
Outbreak defined as 3 or more related cases.7
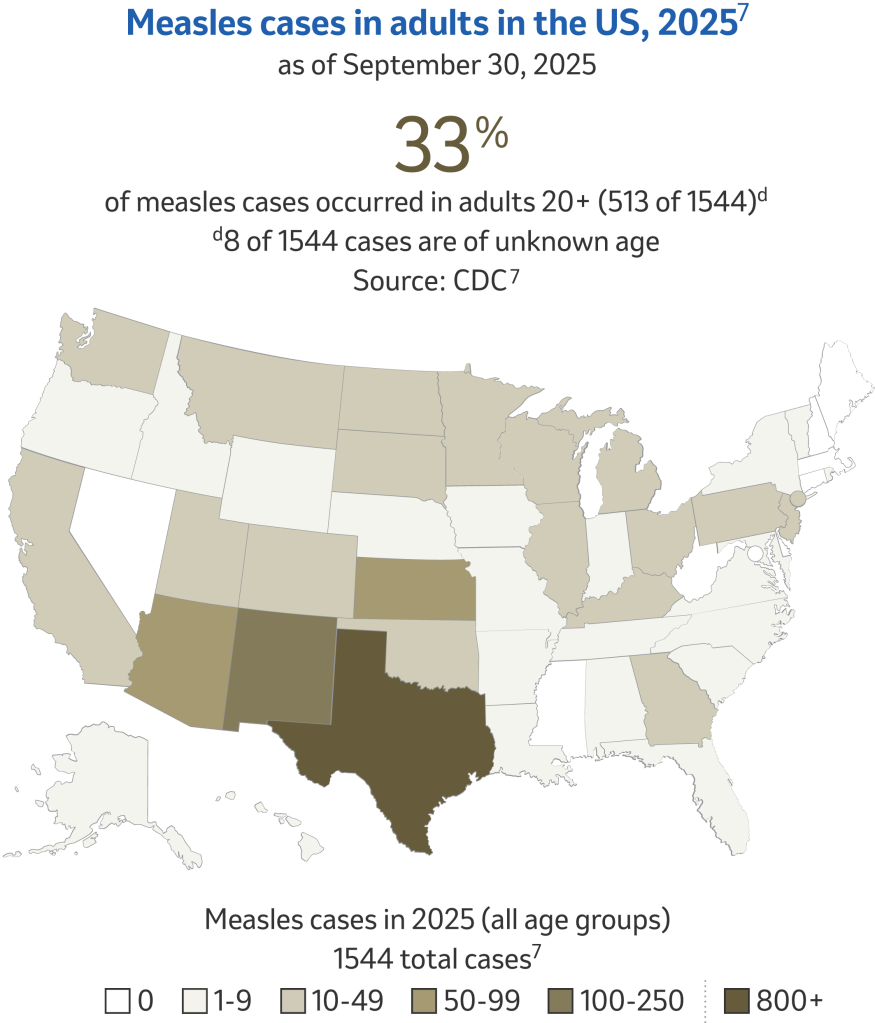
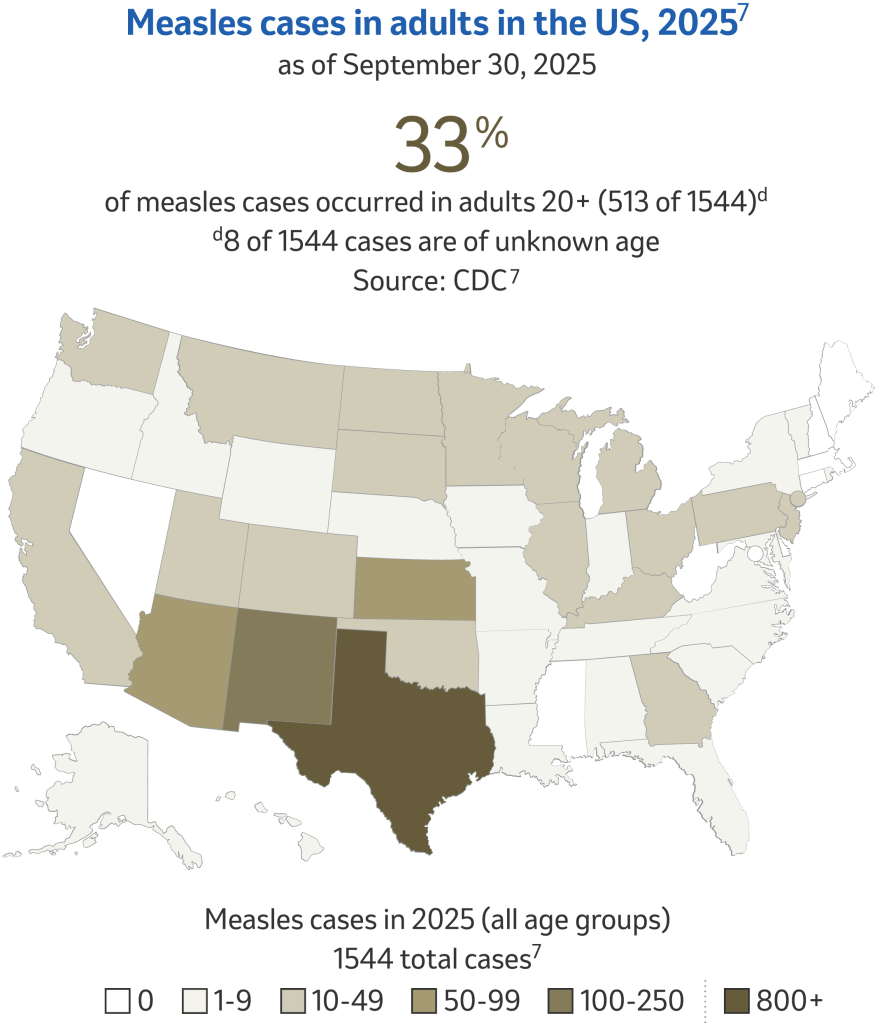
Why is there still a risk for measles?6
Overall measles outbreak risk to the general population is low; however, measles cases have increased globally, increasing the chance of importations into the US.6

Coverage of 95% or greater with 2 doses of
measles-containing vaccine is needed to protect communities from outbreaks. Currently, the global coverage rate of the first dose of measles vaccine is 83% and the second dose is 74%, contributing to a very high number of outbreaks across the world.
– The World Health Organization, April 2024.8

The US reported recent measles vaccination coverage is below 95%.9 During the 2023-24 school year, MMR coverage was 92.7% amongst kindergartners.10 According to the CDC, measles cases continue to be brought into the United States by travelers who are infected while traveling in other countries and most cases come from unvaccinated US residents; putting our herd immunity at risk.4
References
- Centers for Disease Control and Prevention. Measles Vaccination. Updated January 17, 2025. Accessed October 22, 2025. https://www.cdc.gov/measles/vaccines/index.html
- Centers for Disease Control (CDC). Measles prevention. MMWR Suppl. 1989;38(9):1-18.
- Centers for Disease Control and Prevention. Chapter 13: Measles. Last revised April 24, 2024. Accessed October 22, 2025. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-13-measles.html
- Plan for Travel. Centers for Disease Control and Prevention. Last Revised July 15, 2024. Accessed September 30, 2025. https://www.cdc.gov/measles/plan-for-travel.html
- Centers for Disease Control and Prevention. Why CDC Is Involved with Global Measles. Last revised July 14, 2025. Accessed October 22, 2025. https://www.cdc.gov/global-measles-vaccination/why/index.html
- Morbidity and Mortality Weekly Report (MMWR): Measles — United States, January 1, 2020–March 28, 2024. Centers for Disease Control and Prevention. Last revised April 11, 2024. Accessed September 30, 2025. https://www.cdc.gov/mmwr/volumes/73/wr/mm7314a1.htm
- Centers for Disease Control and Prevention. Measles cases and outbreaks. Updated October 22, 2025. Accessed October 22, 2025. https://www.cdc.gov/measles/data-research/index.html
- Global immunization efforts have saved at least 154 million lives over the past 50 years. World Health Organization. Last revised April 24, 2024. Accessed September 30, 2025. https://www.who.int/news/item/24-04-2024-global-immunization-efforts-have-saved-at-least-154-million-lives-over-the-past-50-years/
- Measles, Mumps, and Rubella FastStats. Centers for Disease Control and Prevention. Last reviewed May 13, 2025. Accessed September 30, 2025. https://cdc.gov/nchs/fastats/measles.htm
- Coverage with Selected Vaccines and Exemption Rates Among Children in Kindergarten – United States, 2023-24 School Year. Centers for Disease Control and Prevention. Last revised October 17, 2024. Accessed September 30, 2025. https://www.cdc.gov/mmwr/volumes/73/wr/mm7341a3.htm

