
2 doses of VARIVAX administered
subcutaneously demonstrated
98% estimated vaccine efficacy against the varicella virus in children
The estimated vaccine efficacy for VARIVAX administered subcutaneously for the 10-year observation period:
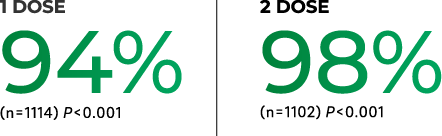

of developing varicella >42 days postvaccination with a 2-dose regimen vs a 1-dose regimen over a 10-year observation period.
a2.2% with 2 doses vs 7.5% with 1 dose.
Study Design
In a clinical trial, a total of 2,216 children 12 months to 12 years of age with a negative history of varicella were randomized to receive either 1 dose of VARIVAX or 2 doses of VARIVAX given 3 months apart. Subjects were actively followed for varicella, any varicella-like illness, or herpes zoster and any exposures to varicella or herpes zoster on an annual basis for 10 years after vaccination. Persistence of varicella-zoster virus antibody was measured annually for 9 years.
IM Administration
Study Design
In an open label clinical trial (NCT00432523), 752 children 12 through 18 months of age received VARIVAX either intramuscularly (n=374) or subcutaneously (n=378), concomitantly with M-M-R®Il (Measles, Mumps, and Rubella Virus Vaccine Live). Ninety-five percent of enrolled children were seronegative to varicella virus at baseline.
Prespecified Primary Analysis Results
In the prespecified primary analysis, seroresponse rates to varicella virus were noninferior in the intramuscular group compared to the subcutaneous group (the lower bound of the 95% CI for the difference in seroresponse rates [intramuscular group minus subcutaneous group] was ≥-10%). The proportions of children achieving antibody titers above the seroresponse thresholds for varicella virus were 88.4% (95% CI: 84.5, 91.6) of children in the intramuscular group and 85.5% (95% Cl: 81.3, 89.0) of children in the subcutaneous group.
- Antibody responses to varicella virus were measured by gpELISA using sera obtained 6 weeks postvaccination.
- Seroresponse rate was defined as the percentage of initially seronegative children who achieved antibody titers above the seroresponse threshold for the assay 6 weeks postvaccination.
- The seroresponse threshold was defined as 5 gpELISA units for anti-varicella virus antibodies.
