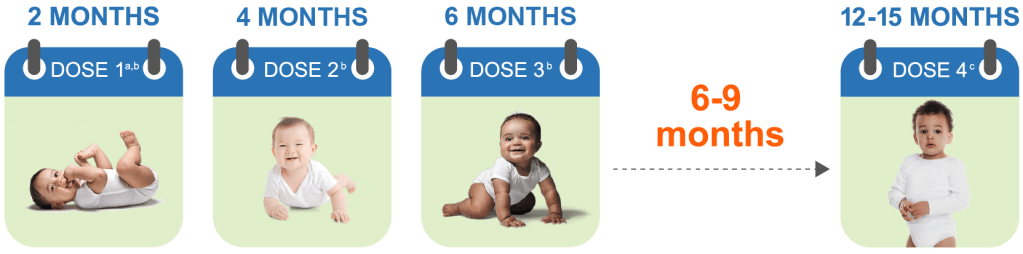
Guidance on pediatric dosage and administration of VAXNEUVANCE
VAXNEUVANCE—a CDC, AAP, and AAFP recommended option for all of your appropriate pediatric patients—is administered as a 4-dose series1-3

CDC recommendation on interchangeability1
VAXNEUVANCE is a CDC recommended option for a 4-dose series or for completion of a 4-dose series initiated with another PCV.
aDose 1 may be given as early as 6 weeks of age.
bThe recommended interval between doses is 4 to 8 weeks.
cThe 4th dose should be administered at approximately 12 through 15 months of age and at least 2 months after the 3rd dose.
How to administer VAXNEUVANCE
VAXNEUVANCE is for intramuscular injection only. Each dose is 0.5 mL.
Hold the prefilled syringe horizontally and shake vigorously immediately prior to use to obtain an opalescent suspension. Do not use the vaccine if it cannot be resuspended. Do not use if particulate matter or discoloration is observed.


VAXNEUVANCE can be given with other routinely administered vaccines (including Pentacel, RECOMBIVAX HB®, RotaTeq®, M-M-R®II, VAQTA®, VARIVAX®, and Hiberix).
Brands mentioned are trademarks of their respective owners.
You have brand choice in the Vaccines for Children Program—choose VAXNEUVANCE for your enrolled patients.5,6
Parents and caregivers of your pediatric patients may be unaware of vaccination programs, such as the VFC Program. VFC is a federally funded vaccine program that provides recommended vaccines to eligible children and adolescents who might not otherwise be able to afford them.6
AAP, American Academy of Pediatrics; AAFP, American Academy of Family Physicians; CDC, Centers for Disease Control and Prevention; PCV, pneumococcal conjugate vaccine; VFC, Vaccines for Children.
Learn about storage & handling information
Review CDC, AAP, and AAFP recommendations
Explore clinical data
See details on reimbursement
References:
- Centers for Disease Control and Prevention. Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2025. Revised October 7, 2025. Accessed October 12, 2025. https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/child/0-18yrs-child-combined-schedule.pdf
- Immunization schedule. American Academy of Pediatrics. Last updated September 17, 2025. Accessed September 30, 2025. AAP.org/immunizationschedule
- American Academy of Family Physicians. Birth Through Age 18 Immunization Schedule. Last updated August 28, 2025. Accessed October 3, 2025. https://www.aafp.org/family-physician/patient-care/prevention-wellness/immunizations-vaccines/immunization-schedules/birth-through-age-18-immunization-schedule.html
- Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines, Annex 3, TRS No 977. World Health Organization. October 19, 2013. Accessed February 26, 2025. https://www.who.int/publications/m/item/pneumococcal-conjugate-vaccines-annex3-trs-977
- Centers for Disease Control and Prevention (CDC). VFC. CDC Vaccine Price List. Updated October 6, 2025. Accessed October 10, 2025. https://www.cdc.gov/vaccines-for-children/php/awardees/current-cdc-vaccine-price-list.html
- Centers for Disease Control and Prevention. About the Vaccines for Children (VFC) Program. Reviewed June 26, 2024. Accessed October 10, 2025. https://www.cdc.gov/vaccines-for-children/about/index.html
