In the absence of clinical efficacy endpoints, vaccine effectiveness against invasive pneumococcal disease may be inferred by comparing immunogenicity of pneumococcal conjugate vaccines to measure (or evaluate) noninferiority in clinical trials.2
The introduction of PCVs has been effective in reducing IPD in children. However, select serotypes—such as Serotype 3, 22F, and 33F—continue to contribute to the burden of disease.4,19
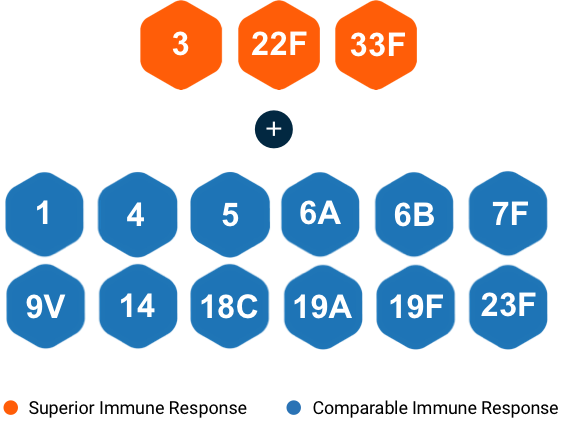
VAXNEUVANCE delivered a robust immune response against 15 serotypes postdose 3 IgG response rates and postdose 4 GMC ratios, includinga:

Comparable immune responses for 12 shared serotypes found in PCV13

Superior immune responses for shared Serotype 3 vs PCV13b,c

Superior immune responses for unique Serotypes 22F and 33F—not covered by PCV1321
Randomized controlled trials assessing the clinical efficacy of VAXNEUVANCE compared to PCV13 have not been conducted.
GMC Ratios, postdose 3a
After a 3-dose primary series, VAXNEUVANCE was found to be noninferior to PCV13 for
12 out of 13 shared serotypes based on the lower bound of the 2-sided 95% CI for the GMC ratio (VAXNEUVANCE/PCV13) being >0.5. For VAXNEUVANCE, the lgG GMC for serotype 6A was just below the noninferiority criteria by a small margin, with the lower bound of the 2-sided 95% CI for the GMC ratio being 0.48 compared to the required criteria of >0.5 for noninferiority.
aMeasurements were taken 30 days postdose specified.
bPostdose 3 IgG response rate percentage point difference vs PCV13, 19.1 (95% CI: 14.4, 24.0).
cPostdose 4 IgG GMC ratio vs PCV13, 1.43 (95% CI: 1.30, 1.57).
Study Design
Study Design
Study 8 was a pivotal, double-blind, active comparator-controlled study in which participants were randomized to receive VAXNEUVANCE (N=860) or PCV13 (N=860) in a 4-dose series. The first 3 doses were administered to infants at 2, 4, and 6 months of age and the fourth dose was administered to children at 12 through 15 months of age. Participants also received other licensed pediatric vaccines concomitantly. Immune responses were measured by IgG response rates, IgG GMCs, and OPA GMTs for all 15 serotypes contained in VAXNEUVANCE.
CI, confidence interval; GMC, geometric mean concentration (mcg/mL); GMT, geometric mean titer;
IgG, Immunoglobulin G; IPD, invasive pneumococcal disease; OPA, opsonophagocytic activity;
PCV, pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine.

Assess immune response vs PCV13

Learn about specific populations

Stay updated on the burden of IPD

View safety & tolerability data
Reference
Reference
Reference
Reference
Reference
Reference
Reference
Reference
Reference
Indications and Usage for VAXNEUVANCE
VAXNEUVANCE is indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F in individuals 6 weeks of age and older.
Select Safety Information for VAXNEUVANCE
Do not administer VAXNEUVANCE to individuals with a severe allergic reaction (eg, anaphylaxis) to any component of VAXNEUVANCE or to diphtheria toxoid.
Some individuals with altered immunocompetence, including those receiving immunosuppressive therapy, may have a reduced immune response to VAXNEUVANCE.
Apnea following intramuscular vaccination has been observed in some infants born prematurely. Vaccination of premature infants should be based on the infant’s medical status and the potential benefits and possible risks.
The most commonly reported solicited adverse reactions in children vaccinated at 2, 4, 6, and 12 through 15 months of age, provided as a range across the 4-dose series, were: irritability (57.3% to 63.4%), somnolence (24.2% to 47.5%), injection-site pain (25.9% to 40.3%), fever ≥38.0°C (13.3% to 20.4%), decreased appetite (14.1% to 19.0%), injection-site induration (13.2% to 15.4%), injection-site erythema (13.7% to 21.4%), and injection-site swelling (11.3% to 13.4%).
The most commonly reported solicited adverse reactions in children 2 through 17 years of age vaccinated with a single dose were: injection-site pain (54.8%), myalgia (23.7%), injection-site swelling (20.9%), injection-site erythema (19.2%), fatigue (15.8%), headache (11.9%), and injection-site induration (6.8%).
The reported solicited adverse reactions in children 7 through 11 months of age who received 3 doses of VAXNEUVANCE were: fever ≥38.0°C (21.9%), irritability (32.8%), injection-site erythema (28.1%), somnolence (21.9%), injection-site swelling (18.8%), injection-site pain (18.8%), injection-site induration (17.2%), decreased appetite (15.6%), and urticaria (1.6%).
The reported solicited adverse reactions in children 12 through 23 months of age who received 2 doses of VAXNEUVANCE were: fever ≥38.0°C (11.3%), irritability (35.5%), injection-site pain (33.9%), somnolence (24.2%), decreased appetite (22.6%), injection-site erythema (21.0%), injection-site swelling (14.5%), and injection-site induration (8.1%).
Vaccination with VAXNEUVANCE may not protect all vaccine recipients.
Before administering VAXNEUVANCE, please read the accompanying Prescribing Information. The Patient Information also is available.
Indications and Usage for VAXNEUVANCE
VAXNEUVANCE is indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F in individuals 6 weeks of age and older.
VAXNEUVANCE is indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae
VAXNEUVANCE is indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F in individuals 6 weeks of age and older.
Select Safety Information for VAXNEUVANCE
Do not administer VAXNEUVANCE to individuals with a severe allergic reaction (eg, anaphylaxis) to any component of VAXNEUVANCE or to diphtheria toxoid.
Some individuals with altered immunocompetence, including those receiving immunosuppressive therapy, may have a reduced immune response to VAXNEUVANCE.
Apnea following intramuscular vaccination has been observed in some infants born prematurely. Vaccination of premature infants should be based on the infant’s medical status and the potential benefits and possible risks.
The most commonly reported solicited adverse reactions in children vaccinated at 2, 4, 6, and 12 through 15 months of age, provided as a range across the 4-dose series, were: irritability (57.3% to 63.4%), somnolence (24.2% to 47.5%), injection-site pain (25.9% to 40.3%), fever ≥38.0°C (13.3% to 20.4%), decreased appetite (14.1% to 19.0%), injection-site induration (13.2% to 15.4%), injection-site erythema (13.7% to 21.4%), and injection-site swelling (11.3% to 13.4%).
The most commonly reported solicited adverse reactions in children 2 through 17 years of age vaccinated with a single dose were: injection-site pain (54.8%), myalgia (23.7%), injection-site swelling (20.9%), injection-site erythema (19.2%), fatigue (15.8%), headache (11.9%), and injection-site induration (6.8%).
The reported solicited adverse reactions in children 7 through 11 months of age who received 3 doses of VAXNEUVANCE were: fever ≥38.0°C (21.9%), irritability (32.8%), injection-site erythema (28.1%), somnolence (21.9%), injection-site swelling (18.8%), injection-site pain (18.8%), injection-site induration (17.2%), decreased appetite (15.6%), and urticaria (1.6%).
The reported solicited adverse reactions in children 12 through 23 months of age who received 2 doses of VAXNEUVANCE were: fever ≥38.0°C (11.3%), irritability (35.5%), injection-site pain (33.9%), somnolence (24.2%), decreased appetite (22.6%), injection-site erythema (21.0%), injection-site swelling (14.5%), and injection-site induration (8.1%).
Vaccination with VAXNEUVANCE may not protect all vaccine recipients.
Before administering VAXNEUVANCE, please read the accompanying Prescribing Information. The Patient Information also is available.
Do not administer VAXNEUVANCE to individuals with a severe allergic reaction (eg, anaphylaxis) to any component of VAXNEUVANCE or
Do not administer VAXNEUVANCE to individuals with a severe allergic reaction (eg, anaphylaxis) to any component of VAXNEUVANCE or to diphtheria toxoid.
Some individuals with altered immunocompetence, including those receiving immunosuppressive therapy, may have a reduced immune response to VAXNEUVANCE.