
Consider IPD protection when it matters
Early protection matters in the first year of life when the risk of pediatric IPD is the highest1-3
The incidence of IPD in children was ~2x as high in the first year of life than that of children 1-4 years of age combined.1,a
The death rate due to IPD was at least 2x as high in infants than in any other pediatric age group.1,b
aBased on pooled analysis of national-level CDC ABC surveillance data from 2019–2023, representing ~35 million people surveyed annually in 10 states across the US. Regional variations may exist. IPD incidence rates were 10.3 in <1 year, 8.0 in 1 year, 4.6 in 2–4 years, 5.5 in 1–4 years, and 1.4 in 5–17 years.1
bBased on pooled analysis of national-level CDC ABC surveillance data from 2019–2023, representing ~35 million people in 10 states across the US. Regional variations may exist. Rates of death per 100,000 population for ABCs areas were 0.62 in <1 year, 0.15 in 1 year, 0.28 in 2–4 years, and 0.06 in 5–17 years. For each age group during 2019–2023, deaths within the ABCs areas and national estimates, respectively, were in <1 year: ABCs areas: 12 deaths in 1,945,628 persons, national estimate: ~113 deaths in 18,392,796 persons; in 1 year: ABCs areas: 3 deaths in 1,965,119 persons, national estimate: ~28 deaths in 18,625,903 persons; in 2–4 years: ABCs areas: 17 deaths in 6,054,551 persons, national estimate: ~163 deaths in 58,047,830 persons; in 5–17 years: ABCs areas: 18 deaths in 28,391,356 persons, national estimate: ~173 deaths in 272,550,379.1
PCVs are CDC recommended as a 4-dose series2
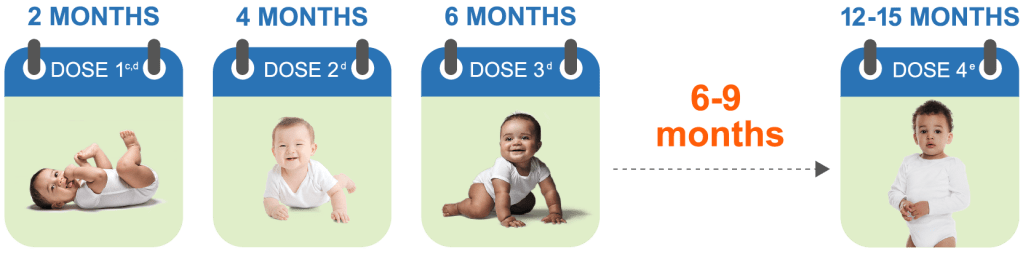

cDose 1 may be given as early as 6 weeks of age.
dThe recommended interval between doses is 4 to 8 weeks.
eThe 4th dose should be administered at approximately 12 through 15 months of age and at least 2 months after the 3rd dose.
Despite the CDC’s recommendation, not all babies complete the 4-dose series.2,4,5,f

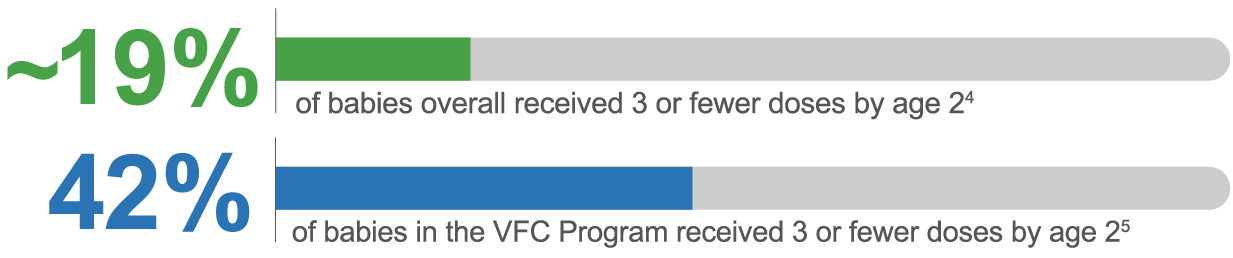
fNIS-Child, a random digit-dialed telephone survey of parents/guardians of children aged 19–35 months that the CDC used to estimate the vaccination coverage with ACIP-recommended vaccines in the US among children born in 2020 and 2021.4
ABC, Active Bacterial Core; ACIP, Advisory Committee on Immunization Practices; CDC, Centers for Disease Control and Prevention; IPD, invasive pneumococcal disease; PCV, pneumococcal conjugate vaccine; NIS-Child, National Immunization Survey – Child; VFC, Vaccines for Children.
Stay updated on serotypes
See immunogenicity data
Learn about special populations
View safety & tolerability data
References:
- Active Bacterial Core surveillance (ABCs). Surveillance reports. Centers for Disease Control and Prevention. Updated August 21, 2025. Last accessed August 28, 2025. https://www.cdc.gov/abcs/reports/index.html
- Centers for Disease Control and Prevention. Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2025. Revised October 7, 2025. Accessed October 12, 2025. https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/child/0-18yrs-child-combined-schedule.pdf
- Gierke R, Wodi P, Kobayashi M. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 17: Pneumococcal disease. Centers for Disease Control and Prevention. Last reviewed August 2021. Accessed May 16, 2025. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-17-pneumococcal-disease.html
- Hill HA, et al. Decline in Vaccination Coverage by Age 24 Months and Vaccination Inequities Among Children Born in 2020 and 2021 — National Immunization Survey-Child, United States, 2021–2023. MMWR Morb Mortal Wkly Rep, pages 844–853.
- Centers for Disease Control and Prevention (CDC). Supplementary Table 1. Estimated vaccination coverage by age 24 months* among children born during 2020-2021,† by selected vaccines and doses and health insurance status§ – National Immunization Survey- Child, United States, 2021-2023. MMWR. 2024;73(38). Accessed January 22, 2025. https://stacks.cdc.gov/view/cdc/162213
