
Choose the robust and early protection of VAXNEUVANCE
VAXNEUVANCE delivers superior immune responses at 7 months for 3 key disease-causing serotypes1,a-c
Superior immune responses for critical disease-causing Serotypes 3, 22F, and 33F1,b-d
Comparable immune responses for 12 shared serotypes
VAXNEUVANCE was studied vs PCV13.
Randomized controlled trials assessing the clinical efficacy of VAXNEUVANCE compared to PCV13 have not been conducted.
No randomized controlled clinical trials have been conducted between PCV20 and VAXNEUVANCE in pediatric patients.
VAXNEUVANCE provides superior immune responses postdose 3 and postdose 4 for Serotype 3a-d
Postdose 3 IgG response rates for Serotype 3 vs PCV13b
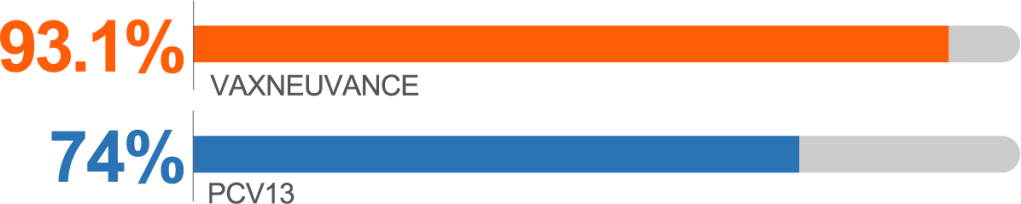
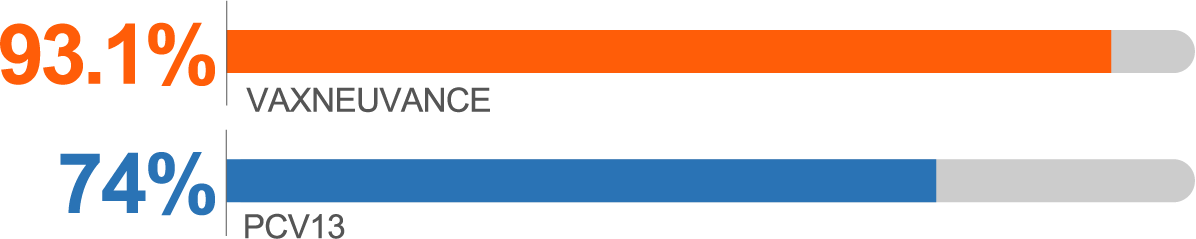
Serotype 3 Postdose 3c
higher immunogenicity vs PCV13
IgG GMC Ratio vs PCV13, 1.70 (95% CI: 1.54, 1.86)
Serotype 3 Postdose 4d
higher immunogenicity vs PCV13
IgG GMC Ratio vs PCV13, 1.43 (95% CI: 1.30, 1.57)
GMC ratios postdose 3a
Primary endpoint: VAXNEUVANCE delivered comparable immune responses for 12 of the 13 shared serotypes found in PCV13. Shared Serotype 6A was just below the noninferiority criteria by a small margin, with the lower bound of the 2-sided 95% CI for the GMC ratio being 0.48 vs >0.5.2
Randomized controlled trials assessing the clinical efficacy of VAXNEUVANCE compared to PCV13 have not been conducted.
Study design
Study 8 was a pivotal, double-blind, active comparator-controlled study in which participants were randomized to receive VAXNEUVANCE (N=860) or PCV13 (N=860) in a 4-dose series. The first 3 doses were administered to infants at 2, 4, and 6 months of age and the fourth dose was administered to children at 12 through 15 months of age. Participants also received other licensed pediatric vaccines concomitantly. Immune responses were measured by IgG response rates, IgG GMCs, and OPA GMTs for all 15 serotypes contained in VAXNEUVANCE.
aMeasurements were taken 30 days postdose specified.
bSecondary endpoint: Postdose 3 IgG response rate percentage point difference vs PCV13 (95% CI): for Serotype 3, 19.1 (14.4, 24.0); for Serotype 22F, 8.1 (5.1, 11.5); for Serotype 33F, -5.1 (-9.5, -0.7).2
cSecondary endpoint: Postdose 3 lgG GMC ratio vs PCV13 (95% CI): for Serotype 3, 1.70 (1.54, 1.86); for Serotype 22F, 3.63 (3.26, 4.04); for Serotype 33F, 1.25 (1.09, 1.44).2
dSecondary endpoint: Postdose 4 IgG GMC ratio vs PCV13 (95% CI): for Serotype 3, 1.43 (1.30, 1.57); for Serotype 22F, 4.77 (4.28, 5.32); for Serotype 33F, 2.68 (2.40, 3.00).2
CI, confidence interval; GMC, geometric mean concentration (mcg/mL); GMT, geometric mean titer; IgG, Immunoglobulin G; OPA, opsonophagocytic activity; PCV13, 13-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine.
Learn about special populations
View safety & tolerability data
Stay updated on serotypes
Get details on early disease protection
References:
- Centers for Disease Control and Prevention (CDC). 2019–2023 serotype data for invasive pneumococcal disease cases by age group from Active Bacterial Core surveillance. Updated March 14, 2025. Accessed August 26, 2025. data.cdc.gov/d/qvzb-qs6p/visualization
- Lupinacci R, Rupp R, Wittawatmongkol O, et al. A phase 3, multicenter, randomized, double-blind, active-comparator controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (PNEU-PED). Vaccine. 2023;41(5):1142-1152. doi:10.1016/j.vaccine.2022.12.054
