
A flexible dosing schedule with RotaTeq® (Rotavirus Vaccine, Live, Oral, Pentavalent)
Vaccination schedule for RotaTeq1,2
Coverage with RotaTeq combines a flexible dosing schedule along side three fully liquid, ready-to-use doses to help protect your pediatric patients.a
The 3-dose schedule of RotaTeq aligns with routine well-baby visits1,3
aCan be completed as early as 14 weeks of age (doses given at 6, 10, and 14 weeks of age). Second and third doses should be given at 4-10 week intervals after previous dose. The third dose should not be given after 32 weeks of age. The safety and efficacy of RotaTeq have not been established in infants less than 6 weeks of age or greater than 32 weeks of age.
Administration of RotaTeq, a rotavirus vaccine
RotaTeq comes in a fully liquid, ready-to-use tube. In clinical trials, RotaTeq was routinely administered concomitantly with other licensed pediatric vaccines.b
bIn clinical trials, RotaTeq was administered concomitantly with diphtheria and tetanus toxoids and acellular pertussis (DTaP), inactivated poliovirus vaccine (IPV), H. influenzae type b conjugate (Hib), hepatitis B vaccine, and pneumococcal conjugate vaccine.


RotaTeq is for oral use only. Not for injection.
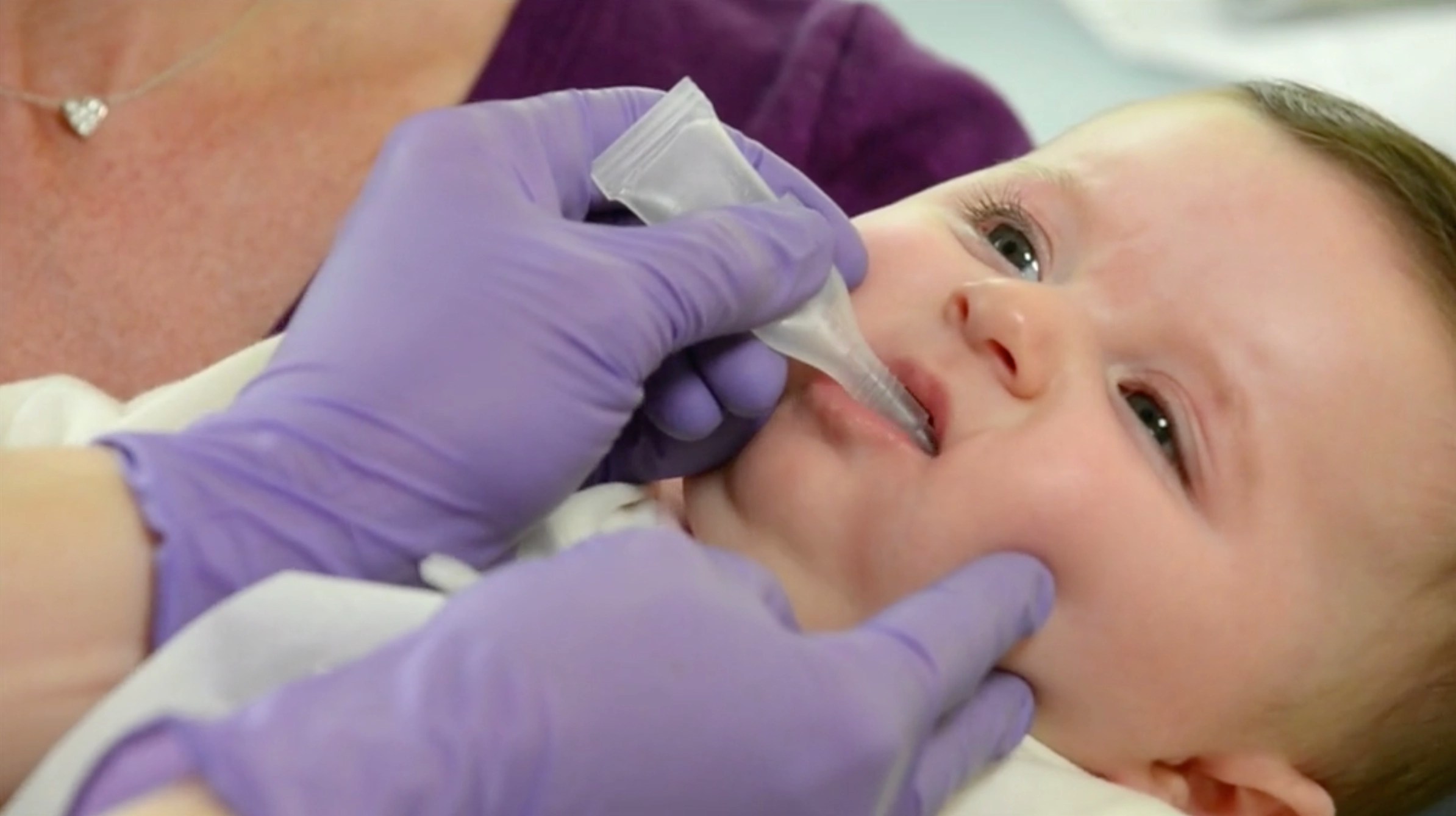
How to administer


Looking to stock RotaTeq in your practice?
Simply register or log in to your account to get started.

ACIP recommendations
Find out about the ACIP recommendations for rotavirus vaccination in pediatric patients.1
Storage and handling
Find out how to store and handle RotaTeq in your practice.
References
- Advisory Committee on Immunization Practices (ACIP). Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2024. Accessed May 29, 2024. https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf
- MedlinePlus. National Library of Medicine (NLM). Well-child visits. Last reviewed January 24, 2023. Accessed May 29, 2024. https://medlineplus.gov/ency/article/001928.htm
- Centers for Disease Control and Prevention (CDC). Rotavirus. Last updated April 25, 2024. Accessed August 18, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-19-rotavirus.html

